The concentration of ammonia in the compound can be determined by using a two cylinder gas jars as shown in diagram 12.
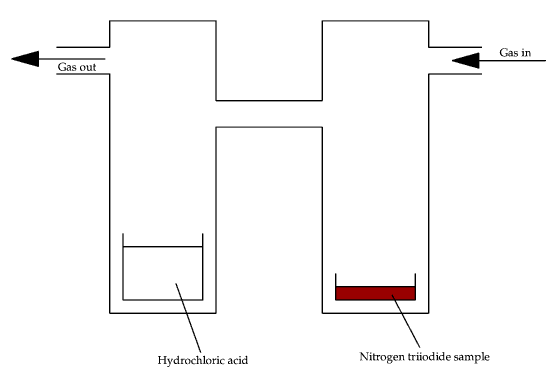
Diagram 12 - Experimental system for determination of ammonia in the compound
The gas (any inert gas will do, helium was used) flows in over the NI3 sample at atmospheric pressure. The gas is light enough to be a carrier and does not allow ammonium chloride fumes (from the reaction of the evolved ammonia and hydrochloric acid) to return to the sample chamber. This ammonium chloride is carried off into a suitable removal system (such as into a fume cupboard).
The flow of gas also keeps the overall pressure constant within the chambers. The constant pressure is required so that the rate of evolution of ammonia will not be greatly altered (which it would if there were any returned ammonium chloride). The pressure also has to be kept constant to prevent explosion of the sample.
The ammonia gas is carried through the system and reacts with the previously standardised hydrochloric acid. The reaction stoichiometry is 1:1. By titrating the acid after (say) 15 hours, the amount of ammonia evolved can be calculated using equations (24) and (25)
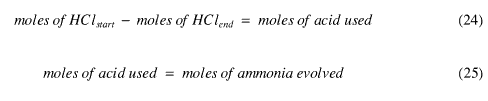
From this relationship, the content of the amount of ammonia in the compound can be found as the breakdown of NI3 is only found when all of the ammonia has desorbed from the compound. This is directly related to the number of ammonia molecules associated with each NI3 molecule.
Pages
- Theory
- Safety
- Theory
- Bomb Calorimetry
- NI3 & Agar
- Analytical techniques
- Chemical Safety
- Practical methodology
- Sound as a detonator
- Determination of ammonia
- Main results
- Method 1 Results
- Ammonia results
- Minimum ammonia results
- Bomb calorimetry results
- Gel times
- IR Results
- XRD Results
- UV Results
- Conversion to HTML
- Bibliography
- Further work
- Acknowledgements
- Technology Used